IMPURITIES
- Expert statements on impurities
- Impurity and degradation profiling
- Evaluation of degradation pathways
- Expertise in solid state degradation
- Management of oos events
- Route cause analysis, CAPAs, etc.
- in silico mutagenicity assessments
- Stability by Design
REGULATORY
- Regulatory advice for CMC
- Expert statements on CMC
- Chinese DMFs (one-stop-shop)
- Scientific advices (FDA, EMA)
- DMF, IMPD, CTD module 3
- Risk Assessment Element. Imurities
- Risk Assessment Nitrosamines
- FDA compliance of documentation
- Combination products
- Environmental Risk Assessment
- Due Diligences
- GMP Audit
CONCEPTS
- IP strategies
- Drug discovery
- Drug development
- Project management
- CRO/CMO identification
- In-depth market knowledge
- Innovative scientific concepts
News

We are hiring!
Project Manager Regulatory CMC (m/f/d)
(20 hours part time)
- RD&C Team

DR. Helmut Buschmann published with Prof. Ulrike Holzgrabe in DAZ
Deutsche Apotheke Zeitung | 159. Jahrgang | 10.01.2019 | Nr. 1/2
- RD&C Team

Risk Assessment Nitrosamine
EMA and edqm ask MAH to evaluate the risk of nitrosamines in marketed synthetic drugs. Risk assessment has to be conducted for drug substance, excipients and drug product. RD&C will assist clients.
- RD&C Team

Prof. Holzgrabe and Dr. Buschmann published in DAZ
Prof. Ulrike Holzgrabe (University Wuerzburg) and Helmut Buschmann (RD&C) published a recent article in DAZ on Remifentanil
- Dr. Helmut Buschmann
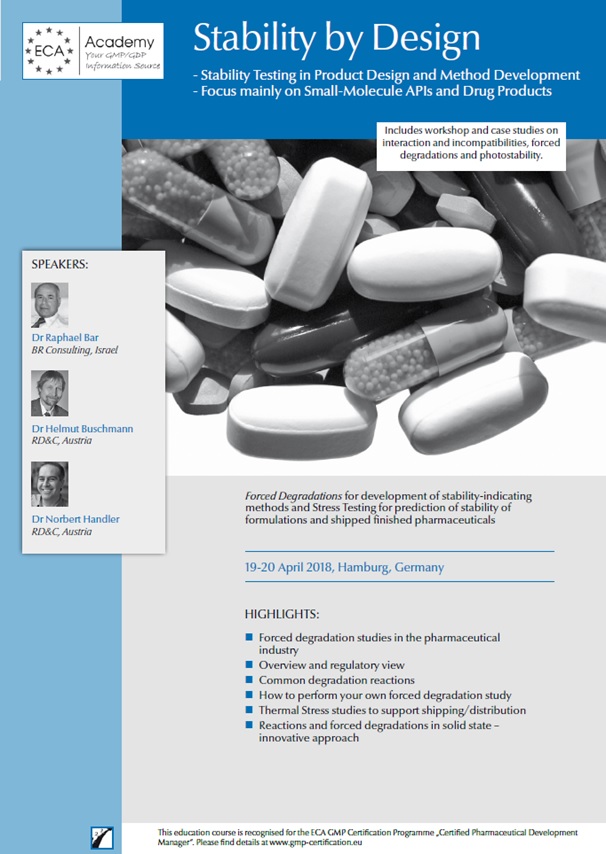
RD&C as invited speakers for 2-days-workshop “Stability by Design”
Concept Heidelberg/ECA invited Helmut Buschmann and Norbert Handler to give talks for the workshop "Stability by Design". The 2-day course will be held in Hamburg on April 19th to 20th 2018.
- RD&C team
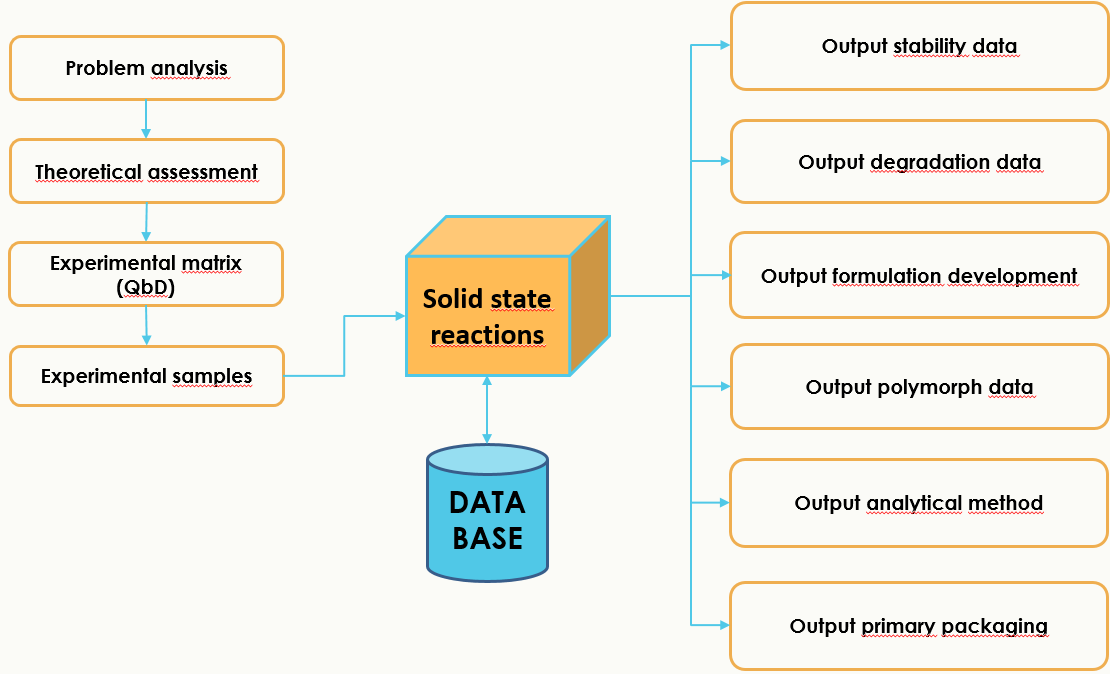
SOLID STATE REACTION PLATFORM
The method could be used for prediction of stability, polymorph changes, shelf-life, degradations in solid environment as well as development tool for solid formulations. The service is offered to customers, but is also open for out-licensing.
- RD&C Team

RD&C GmbH registered the Austrian trade „CONSULTING ENGINEER FOR PHARMACY (Ingenieurbüro für Pharmazie)”.
The strictly regulated trade "Consulting Engineer" warrants a broad technical expert background with an objective view, directed towards protecting the clients’ interests and ensuring superior performance at the lowest commensurate cost. It includes an expert status in the field ensuring quality, employability and professional recognition.
- Dr. Norbert Handler

Dr. Norbert HANDLER is announced “general authorized and certified expert for pharamceutical chemistry” at the Trade Court of Vienna
The expert status is announced for 5 years comprises the fields pharmaceutical chemistry, pharmaceutical products and cosmetics.
- RD&C team

DMF Applications in China
RD&C can act as one-stop-shop for your DMF-application at NMPA in China (including translation, contact to authorities, registration account, regulatory support). Please contact us for more information.
- RD&C team